HPC Simulation for an Endourethral Sphincter against Urinary Incontinence
RELIEF recently proposed a highly innovative Artificial Urinary Sphincter (AUS) against urinary incontinence. The company demonstrated the feasibility, acceptability, and easy use of AUS, but also pointed out the need for optimization of its design, in particular related to the anatomy of the male/female urethra. In this context, HPC-FE simulations can give a disruptive boost to the final development of the product, accelerating the go-to-market strategy, reducing the cost of prototyping and, moreover, minimizing experimental tests.
SECTOR: Healthcare, Manufacturing
TECHNOLOGY USED: HPC, FEM Simulations
COUNTRY: Italy
The challenge
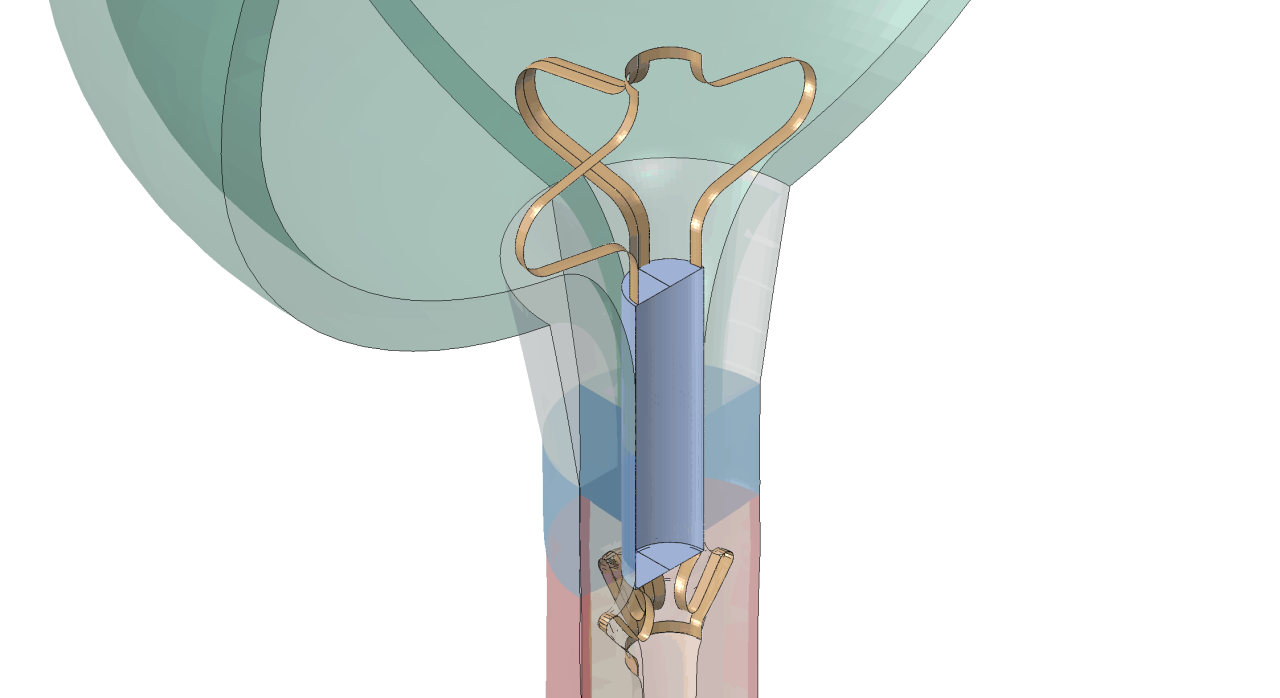
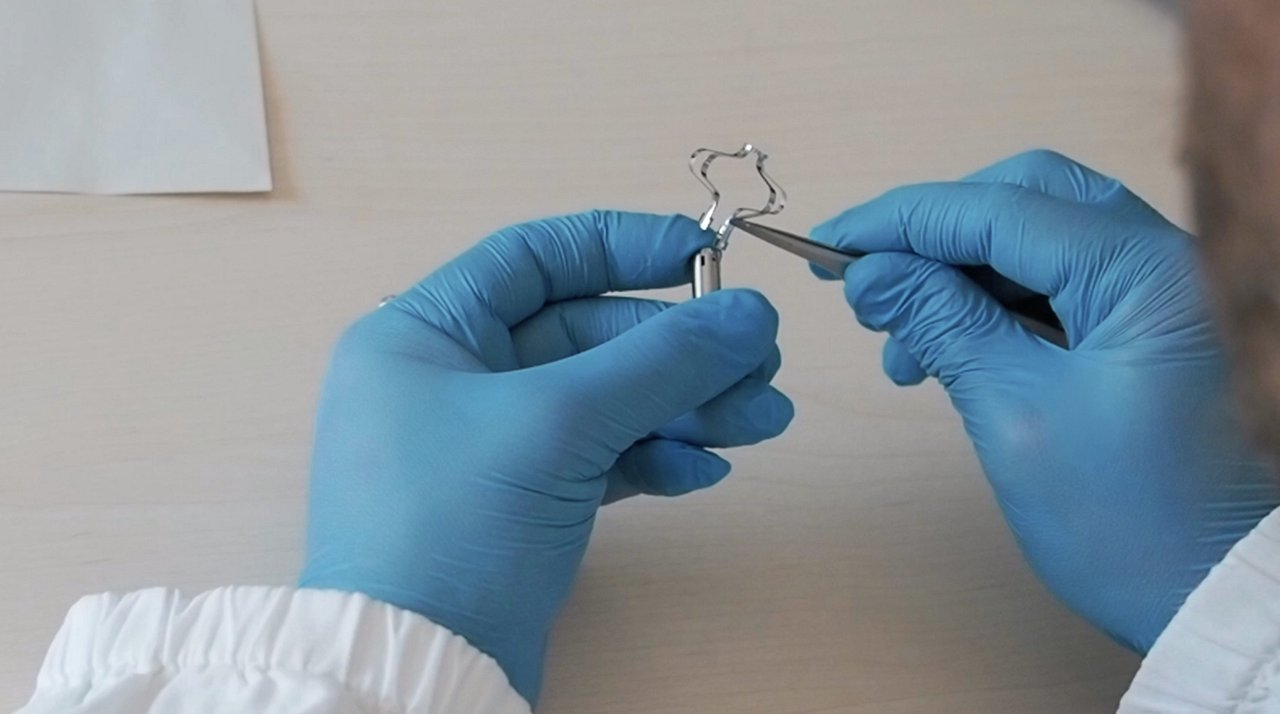
RELIEF has developed an innovative Artificial Urinary Sphincter (AUS), a device for the treatment of incontinence. This disruptive product is the first miniaturised, unisex, minimally invasive, low-cost, and patient-compliant endoluminal artificial sphincter, with advantages over competitive solutions worldwide. The device is not visible outside of the body and completely restores urination control, even in patients suffering from severe forms of urinary incontinence.
In vitro, in-vivo and a first pilot study demonstrated the feasibility, acceptability, and easy use of the AUS, but also the need for optimization of the final product design to avoid migration in the bladder or urethra, to reduce negative long-term side effects on tissue, and to enable patient-specific customization that also takes into account anatomical variability between individuals (both male and female).
To assist with commercialising its AUS, a decision-support system (DSS) is needed to allow medical doctors to choose the optimal size of the device for each individual patient. Until now, RELIEF has been trying to achieve this through a trial-and-error procedure involving a huge effort in terms of cost, time, and ethical issues. consequently, the current methodology only permits the evaluation of a limited number of different conditions.
The solution
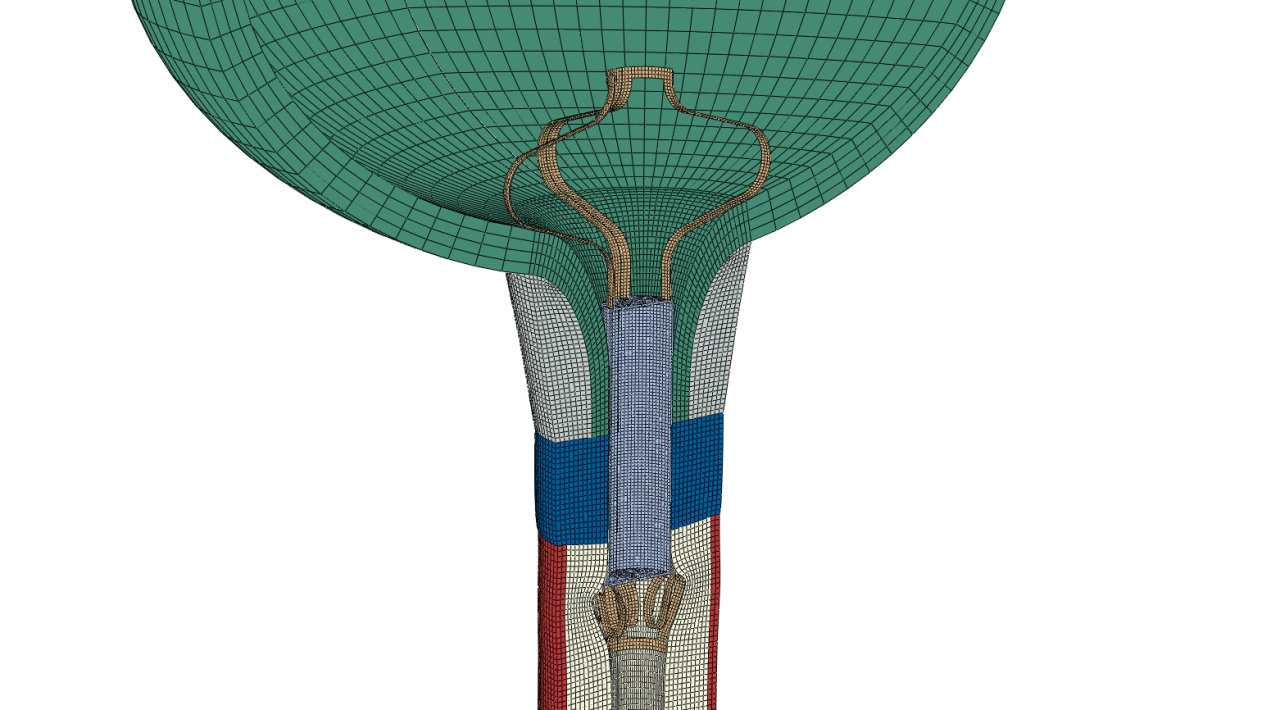
“In silico” investigation by means of numerical simulation gives a disruptive boost to the development of the product, accelerates the go-to-market strategy, reduces the cost of prototyping and, moreover, minimises experimental tests on animal models and human cadavers.
Finite element analyses can be used to optimise the AUS shape design and also to define the key information required to select the device for individual patients. Thus, the side-effects of implantation will be limited and medical doctors can easily choose the right size for a patient, whose long-term satisfaction could be considerably improved. HPC-based simulations mean that the highly complex models, needed to deliver results with sufficient accuracy, are available within RELIEF’s business workflow.
Business impact, Social impact, Environmental impact
“In silico” investigation using numerical simulation gives RELIEF a disruptive boost in the development of the product via:
- Savings in design costs. A reduction of costs by EUR 400 per device is expected. This sums up to more than €40,000 yearly.
- Savings in experiment costs. Numerical simulations, powered by HPC systems, can reduce in-vivo experiments costs by up to 43%.
- Faster time-to-market. This solution reduces the number of prototypes to be designed, manufactured, and tested.
- A competitive advantage. RELIEF has built a DSS that allows medical doctors to easily decide the right AUS size for each patient, providing a more advanced service offer for its product.
This solution will also have a social impact considering:
- Reduction of in vivo tests. Digital models reduce the need for animals or cadavers for validation and optimisation purposes.
- Improvement of long-term patient satisfaction. Personalised selection of the AUS size promotes correct use and reduces undesired effects.
Benefits
- Drastic reduction of animal or cadaver tests for experimental validation and optimisation.
- €40,000 yearly reduction of production cost.
- 43% reduction in experimental cost.
- 6 to 12 months reduction in time-to-market.
Organisations involved:
End User and Technology Expert: Relief srl
Application Expert: Centre for Mechanics of Biological Materials of the University of Padova
HPC Expert: M3E srl
HPC Provider: CINECA